Water In A Solid State
H2o: States of Matter
When an object is a solid, its molecules are bundled in a pattern and tin can't move effectually much.
In a liquid, molecules are farther apart, can motility around, and are non arranged in a blueprint.
The movement is what makes a liquid fluid (or pourable) and take the shape of a container information technology is in.
The molecules in a gas are even further autonomously than in a liquid and move freely with no pattern at all.
Get here to come across what the molecules of substances look like as a solid, liquid, and gas.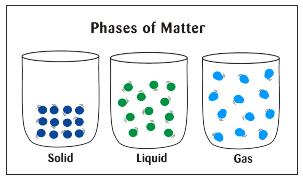
Thing tin can change from one state to some other when concrete conditions change; when energy, such as rut, is added or removed, a substance tin can change from a solid to a liquid, or from a liquid to a gas.
For example, peanut butter does non flow similar a liquid. It acts more like a solid fifty-fifty though it is very soft.
However, if you apply heat (i.e., add energy) to melt peanut butter, its state will change and it volition menses similar a liquid!
Note that non all substances can alter states merely by adding or removing heat—sometimes other physical changes, such equally increased pressure, are needed to alter the state of a substance.
Water is unique because the properties of h2o permit it to exist in all three states of matter!
Water is usually a liquid, but when it reaches to 32° Fahrenheit (F), it freezes into ice.
(Ice is the solid land of water.)
When h2o reaches 212° F, it boils. When it begins to boil, some of the water turns into steam.
(Steam is the gas country of water, and is also chosen water vapor.)
When steam comes into contact with absurd air (which reduces energy), information technology can condense back into water droplets (liquid again).
Those water aerosol could then freeze into (solid) water ice.
Fifty-fifty with all of these state changes, information technology is of import to remember that the substance stays the same—it is all the same water, which consists of two hydrogen atoms and one oxygen atom.
Changing states of matter are simply physical changes; the chemical backdrop of the matter stays the same regardless of its physical state!
Commonly, when water reaches 32° F it begins to freeze.
As you learned in the super-cooled water experiment, water needs a nucleation site, or a spot for the first ice crystals to class.
When there isn't one, water tin reach a temperature below the freezing signal without turning into water ice. When that happens, the water is said to be super-cooled.
Can yous remember of any other ways to continue water from freezing when temperatures are below freezing?
Salt lowers the freezing point of h2o and is often used to melt dangerous water ice off of roads and sidewalks in the wintertime.
To learn more about salt and ice, check out these snow and ice experiments.
To learn more virtually frozen science, see how to make a frozen bubble and super-cooled h2o that freezes in an instant!
Water In A Solid State,
Source: https://learning-center.homesciencetools.com/article/states-of-matter/
Posted by: valdeztrose1977.blogspot.com


0 Response to "Water In A Solid State"
Post a Comment